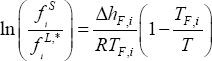Chapter 4:
From Phases to Method (Models) Selection
Abstract
Read the abstractTable of contents
See the table of contentsList of examples
- 4-1: Calculation of the condensation enthalpy of the acetone + water mixture with NRTL at a given pressure (1 bar)
- 4-2: Distribution coefficients in an ideal mixture (propane + n-pentane)
- 4-3: Comparison of phase envelope predictions for the ethane + n-pentane mixture
- 4-4: Behaviour of a methane + n-decane mixture and its models
- 4-5: Behaviour of the benzene + n-hexane mixture and its models
- 4-6: Calculation of the eutectic of para- and ortho-xylene
- 4-7: Comparison of experimental values and different model with H2 + n-hexane mixture
- 4-8: Prediction of a heteroazeotrope with total liquid immiscibility
- 4-9: Formation of hydrates
- 4-10: Example of a vapour-liquid-liquid equilibrium of an acid gas system in the presence of water
- 4-11: VLE and LLE calculation of the methanol + n-hexane mixture
Example 4-6: Calculation of the eutectic of para- and ortho-xylene
Para-xylene is an important chemical stock for the synthesis of terephthalic acid, which is further polymerised to form polyester resins and fibres. Industrial production of pure p xylene is costly, largely due to the difficulties associated with the separation of xylene isomers: they have similar molecular structures and close boiling points, making them difficult to separate by distillation. Nevertheless, their crystallisation behaviours are quite different, as a result of their different molecular structures: para-xylene can pack nicely in a crystalline lattice while ortho-xylene shows steric hindrance. As a result, the melting temperatures and fusion enthalpies have significantly different values (Table 1):
| o-xylene | p-xylene | |
|---|---|---|
| Melting point (K) | 247.98 | 286.41 |
| Heat of fusion at melting point (kJ/mol) | 13.6 | 17.1 |
An additional advantage offered by separation of para-xylene from the liquid through crystallisation is that the solid phase is almost pure. The thermodynamic conditions for this solid-liquid separation must be determined however.
Analysis:
The property to be investigated is the liquid-solid equilibrium, which again means fugacity calculations.
Since the mixture investigated here contains aromatics of equivalent molecular weight, the mixture can be considered ideal.
In addition, pressure is moderate (atmospheric), so that the liquid phase fugacity can be written as:
The solid phase fugacity is calculated, assuming that the heat capacity upon melting,  , and the pressure effect are negligible:
, and the pressure effect are negligible:
Solution:
See complete results in file (xls):
Some help on nomenclature and tips to use this file can be found here.
The pure liquid fugacity is approximated with the vapour pressure, which is extrapolated below the triple point:
At equilibrium, fugacities are equal, meaning:
when para-xylene is crystallising, the liquid phase composition is:
while the o-xylene composition is the complement:
If crystallising from a melt containing large amounts of o-xylene, the solid phase that first appears is pure o-xylene, and the subscripts in the two previous equations must be switched.
The result is shown in figure where the eutectic temperature corresponds to the intersection of the two LSE curves.
 Figure 1 : Eutectic diagram calculation for the binary p-xylene + o-xylene mixture.
Figure 1 : Eutectic diagram calculation for the binary p-xylene + o-xylene mixture.