Chapter 2:
From Fundamental to Properties
Abstract
Read the abstractTable of contents
See the table of contentsList of examples
- 2.1: Refrigeration system
- 2.2: VLE observation
- 2.3: Flexfuel model
- 2.4: Phase envelope of a natural gas with retrograde condensation
- 2.5: Entropy rise in a ideal gas expansion
- 2.6: Cryogenic plant
- 2.7: Distillation column
- 2.8: Energy balance in a column feed
- 2.9: Risk of condensation of water in a gas stream
- 2.10: Effect of the feed composition on the water-gas shift reaction
- 2.11: Effect of temperature on the reaction constant
- 2.12: Chemical looping
Chapter 2 - Abstract
The first item to be investigated when solving a thermodynamic problem concerns the properties: what properties are known and what properties are to be calculated? The scope of this book is not to define these properties exhaustively, nor to derive the relationships among them (many excellent textbooks exist for this purpose [1-4]). However, in order to help the engineer understand how the simulation works, it is essential to summarise the most important relations.
This chapter deals with the “Always True” in thermodynamic simulation: how the fundamental principles are used as tools for modelling concrete problems.
In a first part, a number of definitions will be provided. Gibbs’ phase rule will then be used to help the reader understand the general types of phase behaviour that can be encountered. Finally, Duhem’s phase rule or theorem will help the reader explaining what is the information required in order to make a calculation possible (table 2.7).
| Flash type | Meaning | Applications |
|---|---|---|
| PT | pressure and temperature given | Basic case Used in all calculations |
| Tθ or Pθ | temperature or pressure and vapour fraction given | Bubble point Dew point Partially vaporised flash |
| TV | temperature and volume given | closed vessel at known temperature |
| PH | pressure and enthalpy given | adiabatic distillation columns adiabatic expansions |
| PS | pressure and entropy given | ideal adiabatic compressors pumps turbines |
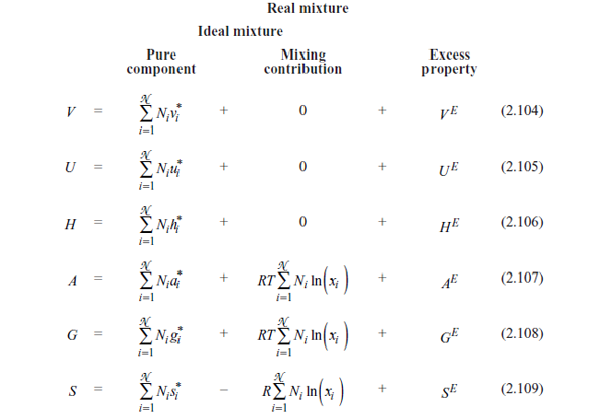
A second part will focus on how these properties are computed. Fundamental thermodynamic relations between properties will be listed. The two types of fluid description (notion of residual property and excess approach) will be given to describe real fluid behaviour (tables 2.14 and 2.15). The notion of activity coefficient will introduce the excess approach. The phase equilibrium will be associated with the equality of chemical potentials or fugacities. Distribution coefficients will be presented to solve various VLE problems like flash, bubble and dew points. The phase equilibrium problem will also be looked at from the point of view of the process, in order to provide a practical guide to the important processes (distillation, extraction, crystallisation, etc.). The presentation of chemical equilibrium will be based on knowledge of the thermodynamic properties.

References
- Smith, J. M., Van Ness, H. C. and Abbott, M. M. “Introduction to Chemical Engineering Thermodynamics”, Sixth Edition; Mc Graw Hill, Inc 2001.
- Elliott, J. R. and Lira, C. T. “Introductory Chemical Engineering Thermodynamics”; Prentice Hall PTR: Upper Saddle River, NJ, 1999.
- Prausnitz, J. M., Lichtenthaler, R. N. and Gomes de Azevedo, E. “Molecular Thermodynamics of Fluid Phase Equilibria”, 3rd Ed.; Prentice Hall Int., 1999.
- O’Connell, J. P. and Haile, J. M. “Thermodynamics: Fundamentals for Applications” 1st Ed.; Cambridge University Press, 2005.
