Chapter 2:
From Fundamental to Properties
Abstract
Read the abstractTable of contents
See the table of contentsList of examples
- 2.1: Refrigeration system
- 2.2: VLE observation
- 2.3: Flexfuel model
- 2.4: Phase envelope of a natural gas with retrograde condensation
- 2.5: Entropy rise in a ideal gas expansion
- 2.6: Cryogenic plant
- 2.7: Distillation column
- 2.8: Energy balance in a column feed
- 2.9: Risk of condensation of water in a gas stream
- 2.10: Effect of the feed composition on the water-gas shift reaction
- 2.11: Effect of temperature on the reaction constant
- 2.12: Chemical looping
Example 2.2: VLE observation
A binary mixture of propane and n-pentane is available at 500 kPa. Using the information on figure 1, indicate the temperature range where vapour- liquid equilibrium can be observed. If a system is known to be at liquid-vapour equilibrium at 333.15 K, which is the proportion of each component in each phase? Can this reading be made on figure 2?
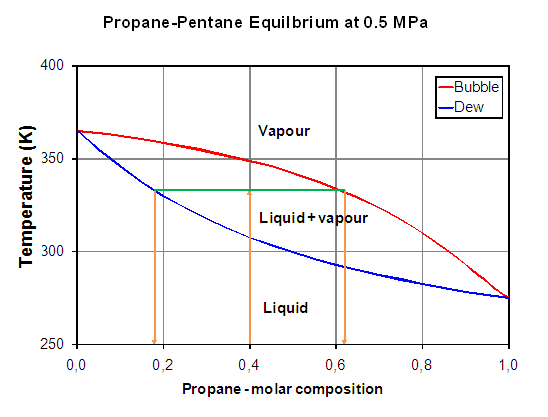 Figure 1: Binary isobar diagram of the propane + pentane system
Figure 1: Binary isobar diagram of the propane + pentane system
 Figure 2: Binary isotherm diagram of the propane + pentane system
Figure 2: Binary isotherm diagram of the propane + pentane system
Analysis:
- The properties to deal with are pressure, temperature and composition.
- The mixture is a binary system with two light hydrocarbons.
- Vapour and liquid are present together at equilibrium conditions.
Solution:
See complete results in file (xls):
Some help on nomenclature and tips to use this file can be found here.
The Gibbs' phase rule indicates that the degree of freedom of the system is 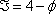 . If two phases are present, two intensive properties are necessary to specify the phase conditions. In a T-xy diagram, pressure is fixed. Hence, a single additional property is required, which can be either temperature, liquid composition or vapour composition.
. If two phases are present, two intensive properties are necessary to specify the phase conditions. In a T-xy diagram, pressure is fixed. Hence, a single additional property is required, which can be either temperature, liquid composition or vapour composition.
The corresponding extreme temperatures at 500 kPa are the boiling points of propane (2 °C on the right of the figure) and of n-butane (96 °C on the opposite side).
At 60 °C, two phases can be observed with a liquid composition of 0.35 and a vapour composition of 0.77 approximately. If composition of the overall mixture is less or greater than these two specified limits, only one phase will be observed. Any mixture with an overall composition included in this range will split into two phases. For example, the 40 % mole propane mixture will lead to the following material balance:
Defining the vapour fraction as  yields:
yields:
This equation is known as the lever rule.
When reading on the Pxy diagram, the compositions obtained are exactly the same.