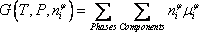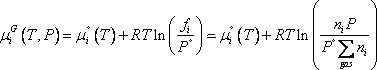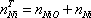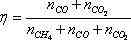Chapter 2:
From Fundamental to Properties
Abstract
Read the abstractTable of contents
See the table of contentsList of examples
- 2.1: Refrigeration system
- 2.2: VLE observation
- 2.3: Flexfuel model
- 2.4: Phase envelope of a natural gas with retrograde condensation
- 2.5: Entropy rise in a ideal gas expansion
- 2.6: Cryogenic plant
- 2.7: Distillation column
- 2.8: Energy balance in a column feed
- 2.9: Risk of condensation of water in a gas stream
- 2.10: Effect of the feed composition on the water-gas shift reaction
- 2.11: Effect of temperature on the reaction constant
- 2.12: Chemical looping
Example 2.12: Chemical looping
Carbon dioxide is one of the main contributors to global warming. CO2 emissions must be reduced to minimise the rise in the earth's temperature. During classical combustion, CO2 is mixed with nitrogen, this dilution making subsequent treatment, such as reinjection, very difficult. One alternative is to carry out selective oxidation of a gas like methane on a metal oxide (e.g. nickel) to produce heat for energy production. The carbon dioxide resulting from this reaction is almost pure and can therefore be captured more easily. The reduced metal can be re-oxidised with air for subsequent chemical looping.
Possible reactions are:
CH4 + NiO = Ni + CO + 2 H2
CH4 + 4 NiO =4 Ni + CO2 + 2 H2O
Analysis:
Operating conditions are atmospheric pressure and temperatures from 800 °C to 1200 °C. The objective is to determine the position of chemical equilibrium. Nickel and nickel oxide are solids while all other components are in the gaseous phase.
As the reaction scheme is not defined (it should change as a function of the temperature), we use the no stoichiometric method to compute the composition. Equilibrium is reached when the Gibbs free energy is minimised.
For solids, the chemical potentials are independent of pressure:
and for gases (with approximation of low pressure):
Gibbs energy of formation as a function of temperature is approximated by a simple linear expression (T in K and Gibbs energy in kcal/mol):
| Component | A | B |
|---|---|---|
| CH4 | -19.662 | 0.024 |
| CO | -26.388 | -0.0216 |
| CO2 | -94.133 | -0.005 |
| H2 | 0 | 0 |
| H2O | -58.442 | 0.0123 |
| Ni | 0 | 0 |
| NiO | -56.714 | 0.021 |
The problem is now to find the minimum of the G function changing the composition of each component. This minimum depends of course on pressure and temperature. Some restrictions may apply to satisfy the conservation of each of the chemical atoms and the molecular proportions of the reacting molecules.
Once the overall quantities of each atom present in the system have been specified, the Gibbs free energy can be minimised for each temperature. Various commercial libraries can be used for this purpose, including the Excel "Solver" add-in. A measure of the level of conversion of methane can be included to monitor the reaction effectiveness:
Results:
See complete results in file (xls):
Some help on nomenclature and tips to use this file can be found here.
Results for optimisations are as follows for two CH4/NiO ratios.
| T (°C) | 800 | 900 | 1000 | 1100 | 1200 |
|---|---|---|---|---|---|
| CH4 | 0.0825 | 0.0311 | 0.0130 | 0.0061 | 0.0032 |
| CO | 0.8912 | 0.9613 | 0.9845 | 0.9930 | 0.9964 |
| CO2 | 0.0263 | 0.0076 | 0.0025 | 0.0009 | 0.0004 |
| H2 | 1.7788 | 1.9144 | 1.9635 | 1.9826 | 1.9909 |
| H2O | 0.0562 | 0.0235 | 0.0105 | 0.0052 | 0.0028 |
| Ni | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| NiO | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Conversion | 91.75 | 96.89 | 98.70 | 99.39 | 99.68 |
| T (°C) | 800 | 900 | 1000 | 1100 | 1200 |
|---|---|---|---|---|---|
| CH4 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| CO | 0.0058 | 0.0090 | 0.0131 | 0.0179 | 0.0234 |
| CO2 | 0.9942 | 0.9910 | 0.9869 | 0.9821 | 0.9766 |
| H2 | 0.0109 | 0.0116 | 0.0124 | 0.0130 | 0.0136 |
| H2O | 1.9891 | 1.9884 | 1.9876 | 1.9870 | 1.9864 |
| Ni | 3.9833 | 3.9794 | 3.9746 | 3.9691 | 3.9630 |
| NiO | 0.0167 | 0.0206 | 0.0254 | 0.0309 | 0.0370 |
| Conversion | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
As we can appreciate from tables 2 and 3, with a CH4/NiO ratio of 1 to 1, all the oxide is reduced. Conversion rises with temperature. If a ratio 1 to 4 was be used, the CH4 would be completely converted and a very small proportion of nickel would remain in NiO form. Other analyses can be carried out changing the CH4/NiO ratio to see the effect on CO and H2 production.
These results are in agreement with the proposed chemical equations below. For NiO/CH4 ratio equal to 1, CO is produced and for a ratio equal to 4, CO2 is obtained. This example shows that the non-stoichiometric approach can also be used with success.