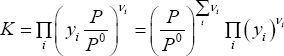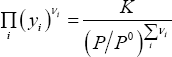Chapter 2:
From Fundamental to Properties
Abstract
Read the abstractTable of contents
See the table of contentsList of examples
- 2.1: Refrigeration system
- 2.2: VLE observation
- 2.3: Flexfuel model
- 2.4: Phase envelope of a natural gas with retrograde condensation
- 2.5: Entropy rise in a ideal gas expansion
- 2.6: Cryogenic plant
- 2.7: Distillation column
- 2.8: Energy balance in a column feed
- 2.9: Risk of condensation of water in a gas stream
- 2.10: Effect of the feed composition on the water-gas shift reaction
- 2.11: Effect of temperature on the reaction constant
- 2.12: Chemical looping
Example 2.10: Effect of the pressure on the methane reforming reaction
In the methane reforming reaction, water and methane react to yield hydrogen gas and carbon monoxide. The reaction is catalyzed so that it can be considered in thermodynamic equilibrium. It occurs in the vapour phase. The equilibrium constant at 800 K is 0.032 if the reference state is taken at 1 bar. What is the effect of pressure on this reaction?
Analysis:
Pressure and temperature are assumed to be known.
The reaction occurs in the vapour phase, and at rather high temperature (this is an endothermic reaction, so its advancement is increased at high temperature). As a first approximation, we can therefore state that the reaction occurs in an ideal gas. The equation that relates pressure with the equilibrium condition is then:
Solution:
See complete results in file (xls):
Some help on nomenclature and tips to use this file can be found here.
The exact stoechiometry of the reaction must first be determined:
The sum of the stoechiometric coefficients,  =3+1-1-1 = 2 is positive (more products than reactants).
=3+1-1-1 = 2 is positive (more products than reactants).
The mole number of each component is written as:
where  is the extent of reaction. The molar fractions are calculated as:
is the extent of reaction. The molar fractions are calculated as:
It is now easy to calculate  , for any pressure, if the extent of reaction is known. In the example sheet, we find this value by numerically solving
, for any pressure, if the extent of reaction is known. In the example sheet, we find this value by numerically solving
Note that  is taken as 1 bar, since the reference state is 1 bar if pressure is expressed in the same unit. The solution is found in figure 1:
is taken as 1 bar, since the reference state is 1 bar if pressure is expressed in the same unit. The solution is found in figure 1:
 Figure 1: Effect of pressure on the extent of reaction
Figure 1: Effect of pressure on the extent of reaction
The conversion decreases with pressure, which is in accordance with the Le Chatelier principle (the system reacts so as to counteract the change that is imposed). Since in the reaction that is considered here more molecules are created than consumed, pressure should result in a decrease of the extent of reaction.