Chapter 3:
From Components
to Models
Abstract
Read the abstractTable of contents
See the table of contentsList of examples
- 3-1: Vapour pressure predictions of methane using different formulas
- 3-2: Quality evaluation of molar volume correlations
- 3-3: Evaluation of the ideal gas heat capacity equations for n-pentane
- 3-4: Quality evaluation of vapourisation enthalpy correlations
- 3-5: Comparison of second virial coefficient calculation methods
- 3-6: Comparison of critical points and acentric factor from different databases
- 3-7: Use of the group contribution methods of Joback and Gani
- 3.8: Diesel fuel characterization
- 3.9: Vapour pressures of di-alcohols
- 3.10: Find the parameters to fit the vapour pressure of ethyl oleate
- 3.11: Fitting of BIP coefficients for the mixture water + MEA with the NRTL model
- 3.12: Separation of n-butane from 1,3 butadiene at 333.15K using vapour-liquid equilibrium
- 3.13: Draw the heteroazeotropic isothermal phase diagram of the binary mixture of water and butanol at 373.15 K
- 3.14: Isothermal phase diagram using the Flory Huggins activity coefficient model
- 3.15: Use of an equation of state for pure component vapour pressure calculations
Example 3-3: Evaluation of the ideal gas heat capacity equations for n-pentane
Two different polynomial (equations (1)) are compared with the DIPPR expression (equation (2)) over the range 200-1500 K. Both are obtained by regression of the DIPPR values, one over the complete range and the other over the range 400-1500 K. The resulting Cp are expressed in J/(kmol.K). Coefficients for the different equations are:
| Equation | A | B | C | D | E |
|---|---|---|---|---|---|
| DIPPR (Aly and Lee) | 88050 | 301100 | 1650.2 | 189200 | 7747.6 |
| Polynomial 400-1500 K | -9352.073 | 516.5751 | -0.3096357 | 9.78224E-05 | -1.3786e-08 |
| Polynomial 200-1500 K | 18576.58 | 366.4169 | -0.03453294 | -0.000109886 | 4.1568e-08 |
The equations are (see chapter 3, section 1.1.2.6):
Analysis:
- The properties are temperature and ideal heat capacity.
- Component is pentane, a databank component.
- The phase is gas at its ideal state.
Solution:
See complete results in file (xls):
Some help on nomenclature and tips to use this file can be found here.
A graph is constructed with the relative difference obtained by the various polynomials and compared against the DIPPR equation (expression of Aly and Lee). The figure shows that is impossible to reproduce the low and high temperatures simultaneously with the polynomial equations. Nevertheless, the fitting is adequate over a shorter range.
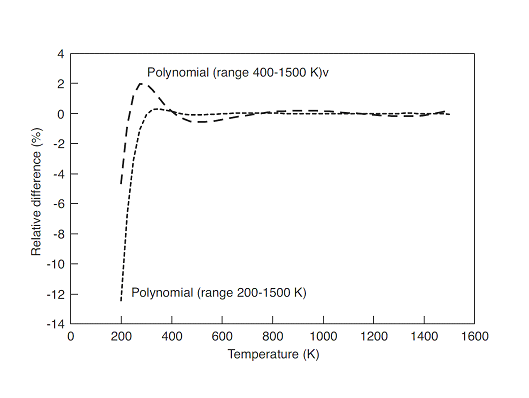 Figure 1: Typical deviation behaviour between the polynomial equations and the Aly and Lee equation for ideal gas heat capacity of pentane.
Figure 1: Typical deviation behaviour between the polynomial equations and the Aly and Lee equation for ideal gas heat capacity of pentane.
References
R. L. Rowley, W. V. Wilding, J. L. Oscarson, Y. Yang, N. A. Zundel, T. E. Daubert, R. P. Danner, DIPPR® Data Compilation of Pure Compound Properties, Design Institute for Physical Properties, AIChE, New York, NY (2003).


